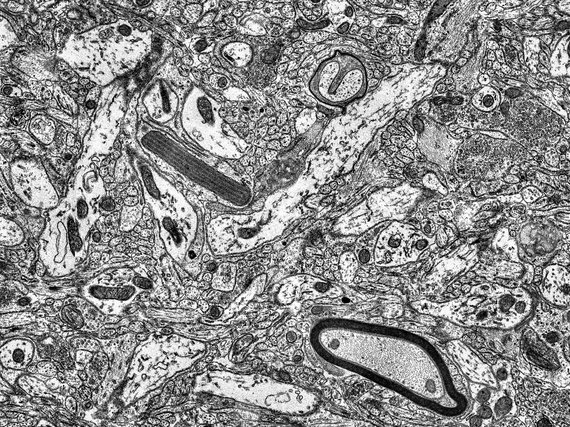
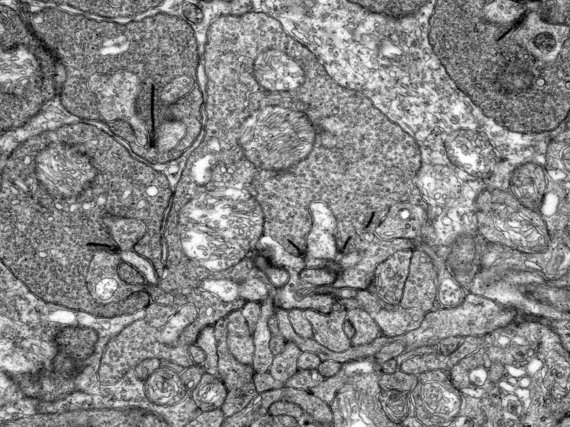
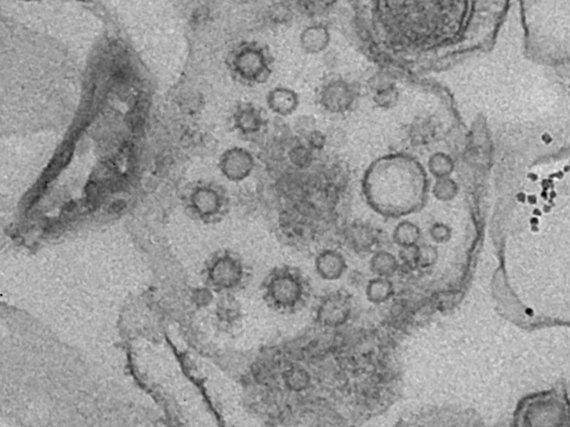
Transmission Electron Microscopy
Transmission electron microscopy is a very powerful tool for the imaging of structures that would not be visible in light and fluorescence microscopy. The main limiting factor of resolution in light microscopy is the wavelength of the light being used: The shorter the wavelenght the better the resolution. The French physicist Louis de Broglie postulated the existence of wave-like behavior of matter and first described the matter-wavelength, now called de-Broglie-wavelength. He won the Nobel Prize for Physics in 1929 after this wave-like behavior was first experimentally demonstrated.
The wavelength of accelerated electrons is up to five orders of magnitude smaller than that of light of common microscopes. The electrons are bent by magnetic lenses in an electron microscope. These lenses can only offer numerical apertures around 100 times smaller than those of light microscopes, leading to a total increase in resolution of around three orders of magnitude.
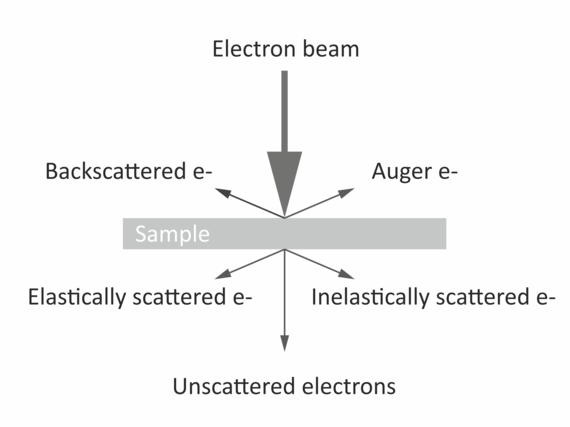
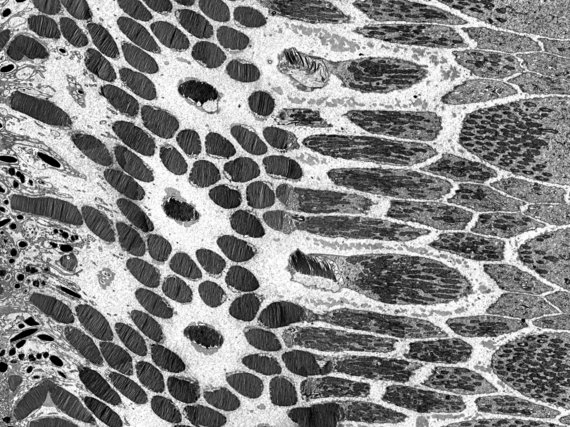
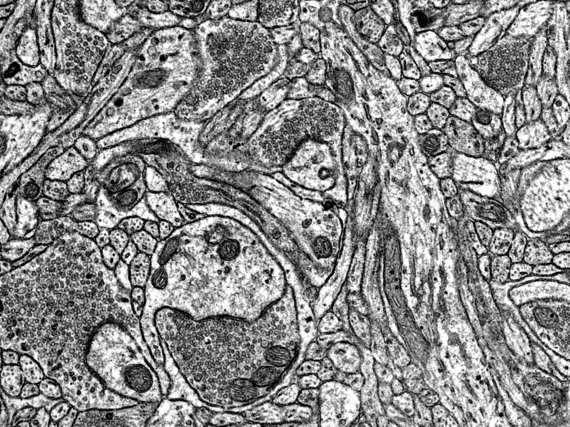
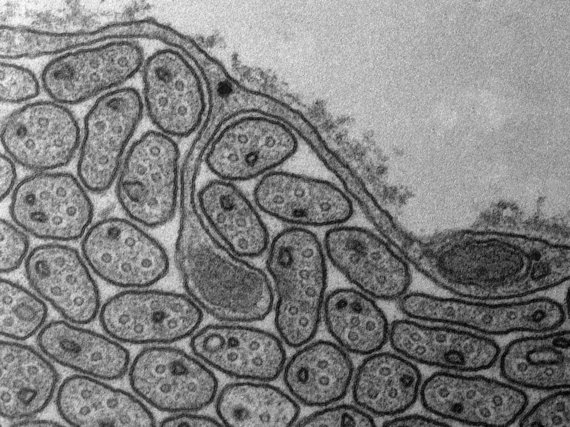
The imaging is based on electron-atom interaction that is dominated by Compton-scattering. The higher the positive charge of the interacting atom, the stronger the effect and thus the stronger generated contrast. Therefore, heavy atoms like gold, osmium, or even uranium are used for staining the samples. The imaging facility provides services such as staining, cutting, and mounting of fixed samples. Once the samples are ready we provide access, training and support on our Zeiss LEO 912B TEM.