New Study Uncovers the Underlying Complexity of Brain Synapses
About the diversity of synaptic connections
A new study recently published in CELL is reshaping our understanding of the fundamental building blocks of the brain, the proteins that are present at synapses. Entitled "The proteomic landscape of synaptic diversity across brain regions and cell types," the research delves deep into the intricate world of synapses, the vital connections between neurons. Led by a team of scientists in the Schuman lab at the Max Planck Institute for Brain Research in Frankfurt, Germany, the study identified over 1,800 proteins that allow different brain synapses to carry out their diverse tasks.
Synapses are the connections between neurons that allow them to communicate with one another. In all cells, including neurons, protein molecules are the main actors who carry out the cell’s work. Synapses are made up of thousands of proteins, each of which plays a unique role in brain function. Brain synapses have diverse functions – from pace-maker like activity to drive brain rhythms to pulsatile properties – and they release different chemicals, “neurotransmitters” and modulators. All of the proteins expressed in a cell or in a cell’s compartment are referred to as its “proteome”. In the present study, the researchers answered a fundamental question: What are the specific proteomes that define the many different types of brain synapses? Scientists have long known that there are different types of synapses, but the specific protein combinations responsible for their diversity have remained a mystery. Understanding the different protein combinations that drive the function of different synapses is fundamental to deciphering brain function and also what goes wrong during disease.
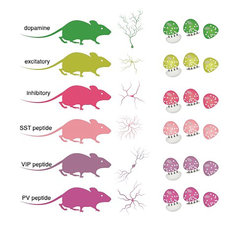
To answer the question of which specific proteomes define different types of synapses, the Schuman team first isolated synapses from different types of neurons in different brain areas. They used genetically engineered mice where the synapses of interest were fluorescently labelled, allowing them to be isolated and purified. Using quantitative mass spectrometry, a method that allows one to identify and quantify the levels of individual proteins, van Oostrum et al. analysed 18 different synapse types in five different brain regions.
In the end, the Schuman team identified more than 1,800 unique synapse type-enriched proteins, revealing a stunning diversity of molecules that underlie synaptic connections. "This represents a significant leap forward in our understanding of synaptic diversity," says van Oostrum. "By unravelling the intricate molecular architecture of synapses, we are not only expanding our knowledge of brain function, but also opening new avenues for research into neurological disorders and potential therapeutic interventions."
The research uncovered common synaptic protein modules that exist at most synapses, but also discovered specific “proteomic hotspots” that drive the specialized function of synapses. For example, in one class of synapses that release the neurotransmitter dopamine, there was a specific depletion of a molecule that helps cells to deal with oxidative stress. Schuman shared: "We are intrigued by this finding given the vulnerability of dopaminergic synapses to oxidative stress and their loss during Parkinson’s disease."
These findings not only provide valuable insights into the fundamental principles of brain function but also open new avenues for research and potential human therapies. Subsequent studies could explore targeted therapeutic interventions, potentially leading to treatments for neurological disorders that are rooted in synaptic dysfunction.












