How is mRNA transported and translated in the neuron?
Güney Akbalik and Erin Schuman (both Max Planck Institute for Brain Research) published a perspective commenting on two research articles by Park et al. (2014) and Buxbaum et al. (2014) published in the latest volume of journal Science on the behaviour of an endogenous mRNA in neuronal processes and fibroblasts. Additionally, they comment on the composition and function of the RNA granule. The papers by Park et al. and Buxbaum et al. describe a transgenic mouse expressing a fluorescently labeled endogenous mRNA, its dynamics and the release of RNA from granules during synaptic plasticity.
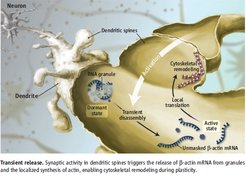
Brain cells (neurons) communicate with up to 10,000 of their neighbors via contact points called synapses. Local protein synthesis at or near synapses is known to be important for long-term memory formation and synaptic plasticity. Since a couple of years, it has become clear that the classical view of protein synthesis in the cell body is not the only way for a neuron to supply proteins to the distal parts, as proteins should be readily available for the fast communication at the synapses.
Messenger RNA molecules, (mRNAs), have been detected in neuronal processes using advanced techniques, such as biochemical analysis, in situ hybridization and RNA sequencing. Though extremely useful to prove the presence of mRNA in the processes, these techniques do not provide any information about the dynamics. In order to observe RNA transport in real-time, one needs to deliver to the cell exogenous fluorescent tags, such as molecular beacons or reporters expressing proteins fused with green fluorescent protein (GFP) which can bind to specific sequences introduced to the mRNA. These techniques, however, interfere with cellular metabolism, often in an invasive way, leading to cell toxicity or even cell death. A solution for these problems is the development of a transgenic mouse expressing an endogenous mRNA with stem loops integrated in the noncoding region and a GFP fusion protein which binds to the stem loops to visualize RNA movement live, as is described by Park et al. Using these mice, the transport rate of the beta-actin mRNA in fibroblasts (around 1.3 micrometers per second) could be determined.
The mRNAs are however not transported as free molecules, but complexed with RNA-binding proteins, in structures called granules. In order to be locally translated into proteins, the RNA molecules must be in an active and free state. Buxbaum et al. found that the release of the beta-actin mRNA from the masked state is directly associated with synaptic plasticity. They proved that the locally available RNA is released from the granule upon induction of neuronal plasticity for translation.
Many important questions are still open, such as whether the properties, described for beta-actin mRNA, apply to all localized RNAs, how many mRNA molecules can be in a granule, if the granules have other roles and how many times a single mRNA molecule can be translated. As labeling the RNA probably does interact with its dynamics, new labeling techniques should be investigated, providing smaller dye molecules.












