Cadherins in 3D live cells
Cadherins are a special class of rod-shaped adhesion molecules, which are essential in embryo development and throughout adulthood. Although binding studies via X-ray crystallography contribute to the understanding of these molecules, only experiments in the living cell can offer the complete picture. By performing live-cell imaging experiments, Sakshi Garg and Erin Schuman from the Max Planck Institute for Brain Research, together with colleagues from the Stelzer Lab at the Biological Sciences Department of the Goethe University, now followed the cell aggregation in vivo and as a function of time by using different types of N-cadherins, including some which were manipulated in such a way that the aggregation is hampered. They published their results in the latest edition of Royal Society Interface.
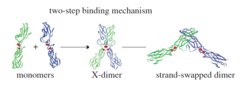
Scheme of the two-step binding mechanism for cadherin trans-interactions. © Royal Society
Cadherins form homodimers with other cadherin molecules present on the same cell (cis-interactions) or on opposing cells (trans-interactions). The trans-binding takes place via a two-step binding mechanism. Although most of the current understanding about interactions of classical cadherins is based on structural biology, the mechanisms in living cells are still mostly unknown. The researchers analyzed the contribution of different N-cadherin binding sites to intercellular adhesion in living cells in a three-dimensional context. Adopting the initial phase of spheroid formation as a model system, they used a combinatorial approach of long-term live-cell imaging and physical-computational modeling. Applying this innovative approach, they showed that in living cells, mutations of the different N-cadherin binding sites affect intercellular adhesion and spheroid formation to various degrees.
They could show that both cis- and trans-dimerization of cadherins are required for spheroid formation, whereby abolishing the trans-interface that is crucial for strand-swap dimerization yields the most severe phenotype. Based on their model, they demonstrated that the probability of junction formation for the strand-swap mutant is highly reduced compared to WT-N-cadherin and the other cadherin mutants. Most interestingly, they pointed out that the mutation of the cis-interface also led to failure of spheroid formation because the intercellular junctions were less stable than for WT-N-cadherin.












